Overview
EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. EGF family members have at least one common structural motif, the EGF domain, which consists of six conserved cysteine residues forming three disulfide bonds. Most are synthesized in membrane-associated pro forms before liberation by proteolytic cleavage. EGF's other family members include HB-EGF,TGF-α, epigen, neuregulins, amphiregulin, betacellulin, and others.
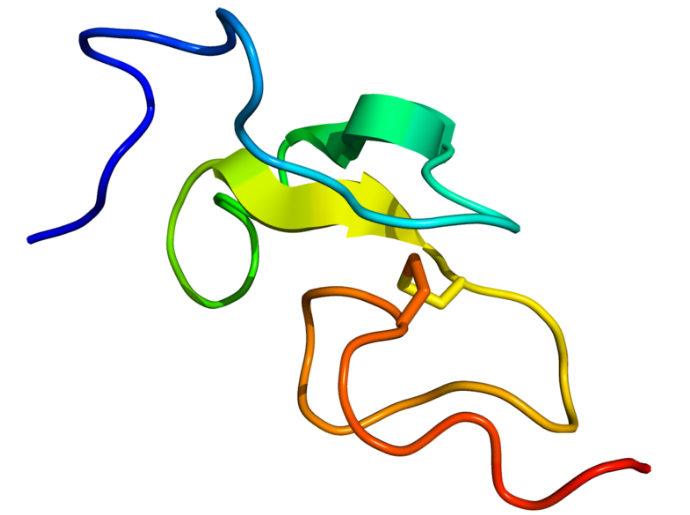
Figure1. EGF protein
Members of EGF family
Table 1. EGF family related products
-
Epidermal growth factor (EGF)
Epidermal growth factor (EGF) stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is a 6-kDa protein with 53 amino acid residues and three intramolecular disulfide bonds. EGF was originally described as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues including submandibular gland, parotid gland. Initially, human EGF was known as urogastrone.
-
Heparin-binding EGF-like growth factor (HB-EGF)
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene. HB-EGF-like growth factor is synthesized as a membrane-anchored mitogenic and chemotactic glycoprotein. An epidermal growth factor will be produced by monocytes and macrophages, as an affinity for heparin is termed HB-EGF. It has been shown to play a role in wound healing, cardiac hypertrophy, and heart development and function.
-
Transforming growth factor-α (TGF-alpha)
Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. It is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling. TGF-α is a transforming growth factor that is a ligand for the epidermal growth factor receptor, which activates a signaling pathway for cell proliferation, differentiation and development. This protein may act as either a transmembrane-bound ligand or a soluble ligand. This gene has been associated with many types of cancers, and it may also be involved in some cases of cleft lip/palate.
-
Amphiregulin (AREG)
Amphiregulin, also known as AREG, is a protein that in humans is encoded by the AREG gene. It is an autocrine growth factor as well as a mitogen for astrocytes, Schwann cells, fibroblasts. It is related to epidermal growth factor (EGF) and transforming growth factor alpha (TGF-alpha). This protein interacts with the epidermal growth factor receptor (EGFR) to promote the growth of normal epithelial cells.
-
Epiregulin (EREG)
Epiregulin consists of 46 amino acid residues. Its secondary structure contains approximately 30 percent of β-sheet in the strand. Some of the residues form loops and turns due to the hydrogen bonding. The percentage of β-sheet in epiregulin depends on the domain and the secondary structures that they occupy. The polymeric molecules of epiregulin have the formula weight of 5280.1 g/mol with a polypeptide(L), a polymer type. Epiregulin can function as a ligand of epidermal growth factor receptor (EGFR), as well as a ligand of most members of the ERBB (v-erb-b2 oncogene homolog) family of tyrosine-kinase receptors.
-
Epigen (EPGN)
Epigen also known as epithelial mitogen is a protein that in humans is encoded by the EPGN gene. Members of this family are ligands for the epidermal growth factor receptor and play a role in cell survival, proliferation and migration. This protein has been reported to have high mitogenic activity but low affinity for its receptor. Expression of this transcript and protein has been reported in cancer specimens of the breast, bladder, and prostate.
-
Betacellulin (BTC)
Betacellulin is a protein that in humans is encoded by the BTC gene located on chromosome 4 at locus 4q13-q21. It is synthesized primarily as a transmembrane precursor, which is then processed to mature molecule by proteolytic events. This protein is a ligand for the EGF receptor. BTC is synthesized in a wide range of adult tissues and in many cultured cells, including smooth muscle cells and epithelial cells. The amino acid sequence of mature mBTC is 82.5%, identical with that of human BTC (hBTC), and both exhibit significant overall similarity with other members of the EGF family.
-
Neuregulins
Neuregulins or neuroregulins are a family of four structurally related proteins that are part of the EGF family of proteins. These proteins have been shown to have diverse functions in the development of the nervous system and play multiple essential roles in vertebrate embryogenesis including: cardiac development, Schwann cell and oligodendrocyte differentiation, some aspects of neuronal development, as well as the formation of neuromuscular synapses.
Cellular functions
EGF family members are best known for their ability to stimulate cell growth and proliferation and are important for many developmental processes including promoting mitogenesis and differentiation of mesenchymal and epithelial cells. EGF can also stop bleeding and accelerate wound healing of skin and mucosa, relieve inflammation, relieve pain and prevent ulcers. The stability of EGF is excellent. It is not easy to lose flow under normal temperature. It can form good coordination effect with various enzymes in human body. The original EGF was mainly used in the medical field, mainly used to promote the repair and regeneration of damaged epidermis, such as burns and scalds.
Role in disease
Recombinant human epidermal growth factor, is used to treat diabetic foot ulcers. It can be given by injection into the wound site, or may be used topically. Tentative evidence shows improved wound healing. EGF is used to modify synthetic scaffolds for manufacturing of bioengineered grafts by emulsion electrospinning or surface modification methods. EGF plays an enhancer role on osteogenic differentiation of dental pulp stem cells (DPSCs) because it is capable of increasing extracellular matrix mineralization. A low concentration of EGF (10 ng/ml) is sufficient to induce morphological and phenotypic changes. These data suggests that DPSCs in combination with EGF could be an effective stem cell-based therapy to bone tissue engineering applications in periodontics and oral implantology. HB-EGF has been shown to play a role in wound healing, cardiac hypertrophy, and heart development and function.
References:
- Stortelers C, Souriau C, van Liempt E, van de Poll ML, van Zoelen EJ. "Role of the N-terminus of epidermal growth factor in ErbB-2/ErbB-3 binding studied by phage display". Biochemistry. 41 (27): 8732–41.